Background
Among the many attributes critical to cannabis and other ingestible products, water content is perhaps the most widely understood. Live plants are comprised mostly of water moisture, and most of this moisture must be removed after harvest of the plant to create a shelf-stable product that will not mold or mildew, to preserve and concentrate desired compounds like terpenes and cannabinoids, and to allow combustion or extraction. This process of removing moisture typically requires multiple days of drying and curing, allowing the moisture to evaporate until the product reaches the critical parameter around <15% moisture.
The state-of-the-art in cannabis moisture testing is a loss-on-drying (LOD) moisture balance. This is a very straightforward technique where a sample is weighed on a scale while heat is applied. As the heat causes the water to evaporate, the weight of the sample decreases, finally reaching a steady value when all the free water has finished evaporating. The amount of water weight lost is then compared to the starting weight to determine the weight fraction of water. This approach is used across nearly all industries, with the primary drawbacks being the time necessary to dry the water (generally tens of minutes per sample), the destruction of the sample from the heating process, and the cost of the moisture balance and its operation.
In addition to this water moisture content, many states require a further measurement that is generally less understood: water activity. Whereas the moisture content is simply the weight of the water in the flower as a percentage of the overall flower weight, water activity is essentially a measure of how this water is distributed. Water activity (aw) ranges from 0 to 1, where values closer to 1 mean the water is on the outside of the flower and able to be dried (“free water”), and values closer to 0 mean the water is molecularly bound within the flower and cannot be dried off (“bound water”). This is important as any water that is not bound within the plant can encourage mold and mildew microbe formation, so it is desirable to remove all of the free water and reach a water activity of 0.65 or less. Typically, there is no microbe growth to be expected if water activity is below 0.6. However, if water activity goes below ~0.55 the terpenes can be damaged and overall quality of the finished product suffers.
Laboratory measurement of water activity is accomplished by different means, from placing the sample in a humidity chamber and measuring the amount of water that evaporates from the sample, to newer laser-based systems that operate similar to the LOD method. As with moisture measurement, the primary drawbacks to these techniques are the tens of minutes required for the measurement, the destruction of the sample, and the cost of the apparatus and operation.
The Purpl PRO by Purpl Scientific, Inc. is a new generation of analytical instrumentation aimed at simplifying these measurements. Based on near-infrared spectroscopy, a technology originally developed for agricultural measurements 70 years ago, and currently used for instantaneous testing of multiple product attributes in nearly every industry, the Purpl PRO is aimed at bringing this well-proven approach to the cannabis industry. In addition to the key attributes of instantaneous, non-destructive testing, the Purpl PRO also aims to simplify this approach by making it portable for use in and around any cultivation or retail environment, affordable for any size operation, and simple in operation to allow accurate testing by any user.
This document details the scientific procedures and results of the Purpl PRO +H2O Pack water measurement validation. It is intended to show the accuracy and repeatability of the Purpl PRO +H2O Pack against traditional cannabis testing laboratories, and to provide data to support the use of the Purpl PRO in measuring moisture and water activity.
Materials & Methods
Development of any new analytical technique is comprised of two primary segments. First, the new technique is calibrated against a known reference method, using a broad range of inputs to sufficiently cover the expected measurement range. Second, the predictions generated by the initial calibration are validated on a new set of unknown samples in a blind fashion, in order to assess the prediction accuracy and repeatability.
Initial: As is standard with near-infrared spectroscopy method development, each sensor was first referenced to a white diffuse reflectance standard to allow measurement of optical absorption. This white referencing was performed on each sensor each day of sample measurement. The optical measurement window and all accessories that contact the cannabis samples were thoroughly cleaned between each measurement to prevent cross-contamination.
Calibration: Initial calibration of the Purpl PRO sensors was performed in certified cannabis testing laboratories in Oregon and Arkansas. A total of 55 flower samples were analyzed for this purpose, including freshly harvested (“wet”) cannabis and hemp flowers as well as dried and cured samples. Samples were either dried in a low temperature oven to reduce native moisture and water activity, or placed in a humidified chamber to increase the native moisture and water activity. These procedures were done to allow sample measurement across the typical full ranges. For Purpl PRO measurement, each sample was ground and mixed for improved homogenization, then distributed across eight Purpl PRO sensors. The sensors recorded at least 4 and up to 30 near-infrared spectra from each sample, and a total of 10,926 near-infrared spectra were obtained.
After the near-infrared scanning, the aliquots of each respective flower sample were recombined within a separate sealed container and mixed for homogenization. These samples were then analyzed by the cannabis testing laboratories via moisture balance and water activity analyzer per the lab’s standard operating procedures. Because water in a sample is affected by the surrounding environment, all measurements were performed as quickly as possible and immediately before laboratory analysis to maximize the prediction accuracy. The sample moisture concentrations, as determined by the laboratory, ranged from 1.3 to 82.2% w/w, and water activity ranged from 0.26 to 0.99
The near-infrared spectra were then correlated to the lab-reported values. An initial multivariate analysis was performed using partial-least-squares (PLS) regression for each of the two water measurements. These PLS models were used to screen the dataset for outliers. After the removal of the outliers, the spectral data were used as inputs to a machine-learning model. These machine-learning prediction algorithms were developed to maximize predictive accuracy, minimize measurement variance, recognize outlier artifacts such as non-cannabis samples, stems, or seeds, and to minimize device-to-device variations. These algorithms were then applied to the Purpl PRO devices via the +H2O Pack.
Validation: 8 Purpl PRO sensors were selected at random to be used at the same cannabis testing laboratories. Samples were ground and homogenized as before, and aliquots of each homogenized sample were distributed across the 8 Purpl PRO sensors. After this measurement, the samples were recombined and analyzed by the laboratory, as above, to compare the predicted results from the Purpl PRO +H2O Pack against the laboratory values.
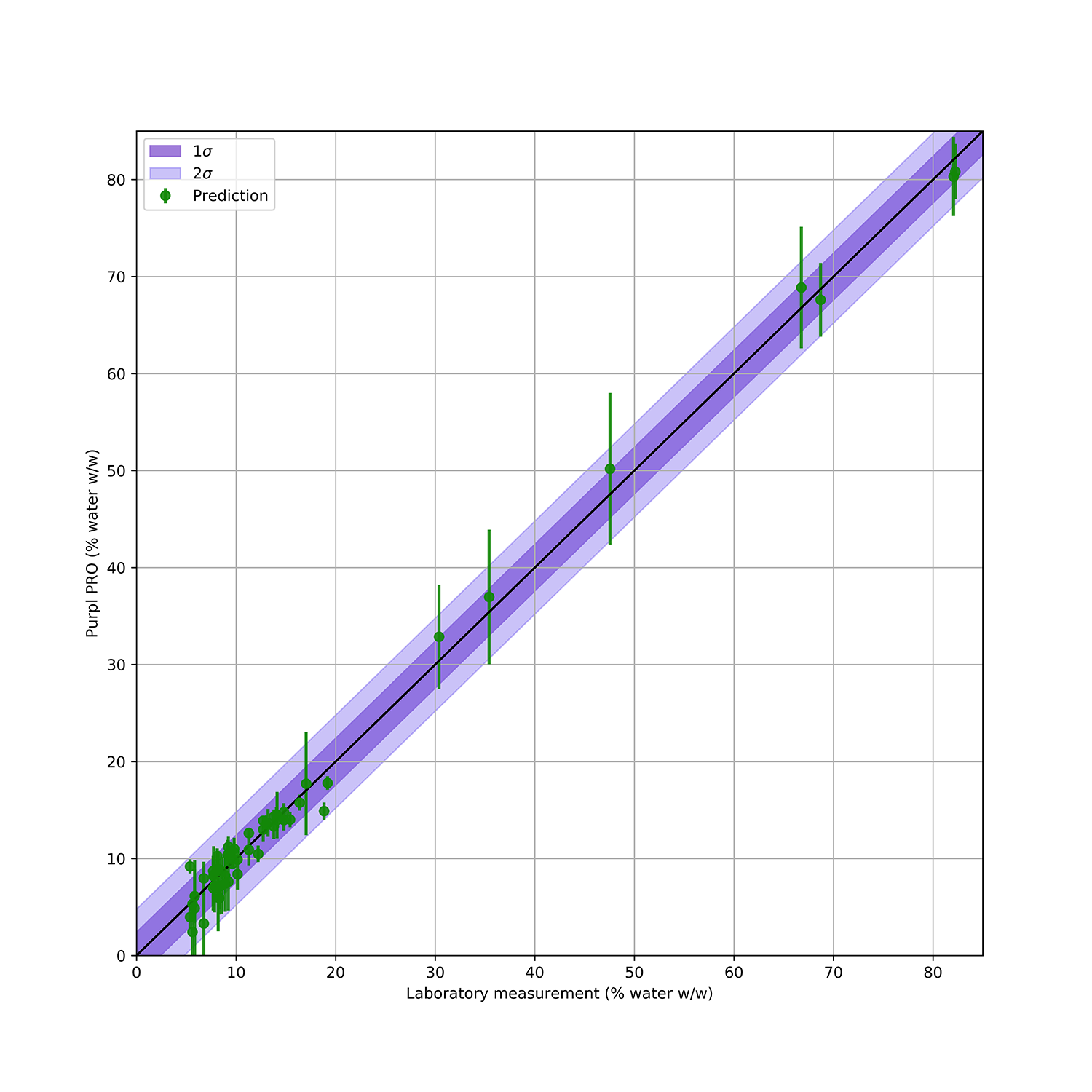
FIGURE 1: Validation of Purpl PRO +H2O Pack moisture measurements (% water) against LOD analysis at two state testing laboratories. Data points indicate average value across 8 sensors, whiskers indicate all measurements. Purple shading indicates standard deviation (dark = 1σ, light = 2σ).
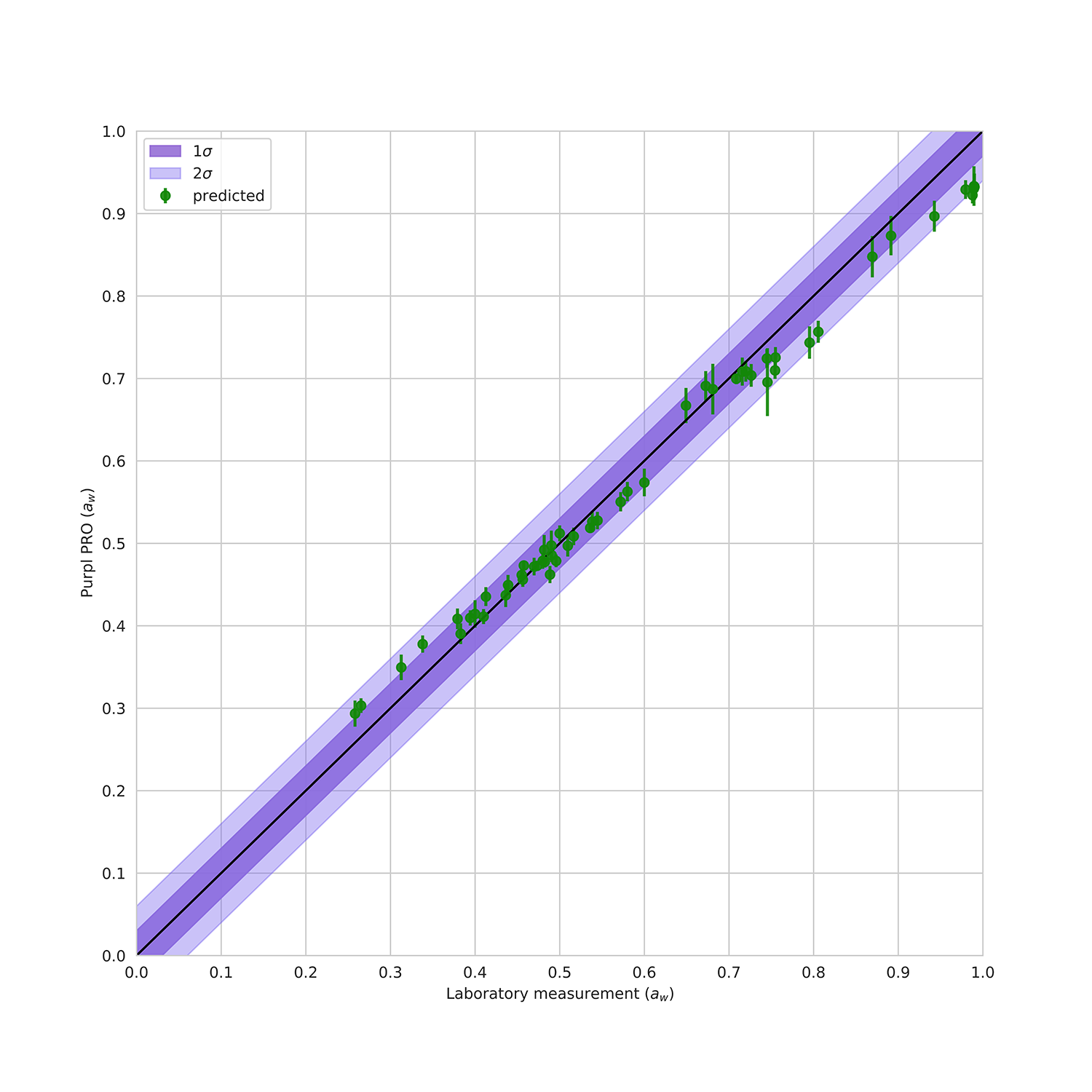
FIGURE 2: Validation of Purpl PRO +H2O Pack water activity (aw) measurements against water activity analysis at two state testing laboratories. Data points indicate average value across 8 sensors, whiskers indicate all measurements. Purple shading indicates standard deviation (dark = 1σ, light = 2σ).
Results
The moisture content predictions of the Purpl PRO +H2O Pack validation measurements are shown in Figure 1. This plot shows the average predicted moisture via the Purpl PRO devices compared to the moisture balance measurements from the testing laboratories. The mean absolute error (MAE) of the Purpl moisture prediction, based on both calibration and validation datasets, is 2.4%. For the critical range of 10-20% moisture, MAE is 1.5%. These prediction errors are comparable to those reported for laboratory LOD analysis of cannabis samples.
The water activity predictions of the Purpl PRO +H2O Pack validation measurements are shown in Figure 2. This plot shows the average predicted water activity (aw) via the Purpl PRO devices compared to the measurements from the respective laboratories. The MAE of the Purpl water activity prediction, based on both calibration and validation datasets, is 0.02. As with the moisture prediction, these errors are comparable to those reported for laboratory water activity analysis.
Conclusions
This study demonstrates the accuracy of the Purpl PRO +H2O Pack for moisture and water activity measurement of cannabis and hemp samples across the ranges typical of the product, from initial harvest through final, cured product. The accuracy of each measurement compares favorably to tests performed by licensed cannabis testing laboratories, but with the significant advantages of instantaneous results, reduced cost, reduced complexity, and the capability to perform tests in the field or any commercial environment.







